Over the last 30 years increases in epizootic diseases, eutrophication of freshwater resources, and environmental pollution have had a negative impact on the production of commercially available Pacific White Shrimp (Litopenaeus vannamei). Traditional production of food shrimp typically utilized outdoor earthen ponds with frequent water exchanges to maintain optimal dissolved oxygen and water quality. These practices increased the probability of release and introduction of pathogen and eutrophication of coastal waters. The use of zero exchange recirculating aquaculture systems (RAS) is an alternative method of shrimp production. It can minimize adverse environmental impacts and improve biosecurity, defined as “the sum of all procedures in place to protect living organisms from contracting, carrying, and spreading diseases and other non-desirable health conditions” (Moss et al., 1998). Significant reduction in released nutrient-rich water allows the system to be operated with native or exotic species with minimal risk of escapement into receiving waters while simultaneously decreasing the probability of disease introduction from water exchange (Moss, 2002). Although zero exchange systems represent an environmentally friendly form of shrimp production, the associated cost is difficult to offset unless super-intensive practices are used (Balasubramanian and Ravachandran, 2004; Stokes et al., 2009; Wasielesky Jr. et al., 2006). These practices decrease the probability of pathogen introduction, increase biosecurity while producing cost-effective margins of biomass production (Browdy and Moss, 2005; Wasielesky Jr. et al., 2006) demonstrated that zero exchange super-intensive methods enclosed in greenhouse structures decrease these risks while allowing for close monitoring of environmental parameters. Further, implementation of super-intensive zero exchange microbial dominated RAS in greenhouse settings resulted in 98% survival and no disease indicating improved biosecurity while increasing biomass compared to shrimp grown in clear water (Wasielesky Jr. et al., 2006). Similar results have been reported by Ballester et al (2010) with slightly lower survival (~89%), however the microbial-based super-intensive systems supported increased shrimp yield while decreasing operation costs compared to non-microbial based super-intensive systems. The results from Wasielesky Jr. et al (2006) and Ballester et al (2010) suggest that the presence of a complex community of microorganisms can remove potential toxicants from the water, promote shrimp growth through feed supplementation and improve shrimp health by preventing allochthonous pathogenic organisms from invading the system through competition.
The utilization of probiotic applications, as defined by Verschuere et al (2000), to RAS has become recognized as a beneficial practice to shrimp aquaculture see review by Ninawe and Selvin, (2009). Generally, a single or combination of several known cultures isolated from aquacultured shrimp or water used for culture is used to inoculate the system. Organisms from the genus Vibrio (Austin et al., 1995; Balcázar et al., 2007), Bacillus (Gómez and Shen, 2008; Li et al., 2007; Rengipat et al., 2003; Vaseeharan and Ramasay, 2003; Solano and Soto, 2006), Pseudomonas (Vijayan et al., 2006), Roseobacter (Sandaa et al., 2003), and Arthrobacter (Li et al., 2006) have all been employed in shrimp aquaculture or display promise as potential probiotics (reviewed by Ninawe and Selvin, 2009). These bacteria once established within a RAS aid in the maintenance of water quality, most often with respect to nitrification (Kuhn et al., 2010). Further advantages to bacterial supplementation include microbial floc as natural food to improve growth (Burford et al., 2004; El-Haroun et al., 2006), prevention of pathogen proliferation through competitive exclusion (Verschuere et al., 2000), and immunostimulation of the immune system of target organisms (Ganguly et al., 2010). Although probiotic cultures have proved beneficial, most organisms rely on a complex community of microbial flora to grow most efficiently and aid in immune function. The use of pre-conditioned (established microbial community) RAS water has yet to become a common practice in aquaculture because of the difficulty to maintain and promote a microbial community which represents the target organism throughout its commercial growth period to food-size.
The current study assesses the efficacy of fully established heterotrophically dominated water from a 62-day nursery trial to act as a natural probiotic community to grow L. vannamei juveniles to food-size in greenhouse-enclosed super-intensive RAS under no water exchange. Further, we analyze the effectiveness of settling and foam fractionation to control microbial and algal communities within the system using flow cytometry.
1 Results
1.1 Shrimp grow-out period
Biometric comparative analysis of settling tank (ST) versus foam fractionator (FF) salinity treatments (30.8 vs. 30.3, respectively) was significantly different throughout the course of this study. In addition, although the alkalinity in each of the raceways was adjusted at least two times per week to maintain a concentration of 160 mg/L as CaCO3, mean alkalinity in the FF raceway was also significantly lower than ST raceways containing 124 vs. 129 mg/L CaCO3. Similarly, we observed significant differences in daily nitrate concentrations in raceways using the FF method (1 027 vs. 855 NO3 mg/L or 232 vs. 193 mg/L NO3-N). On the last day of our study, we observed the FF raceway nitrate concentration at 2 028 NO3 mg/L vs. the ST raceway concentration at 1 585 NO3-N mg/L. We speculate that in the bottom of the ST, denitrification occurred under the anaerobic, sludge-water interface. The mean alkalinity in the total ammonia-nitrogen and nitrite levels in all four raceways remained very low throughout the study (>0.5 mg/L). Suspended solids were consistently maintained between 10 and 30 mL/L, although on Day 43 one raceway reached a concentration of 33 mL/L. We also maintained TSS concentrations at 400 to 500 mg/L and only observed a spike (790 mg/L) once in one raceway over the duration of the study. A large portion of the TSS was in the form of VSS. No signs of bacterial or viral pathogen infections were found in any of the shrimp samples sent to Texas Veterinary Diagnostic Laboratory for analysis. Our results from this study show that 7.5 kg/m of shrimp biomass can be sustained with periodic oxygen supplementation consisting of a mixture of 100% O2 with ambient air at a rate of 1.0 L/min for 30 to 60 min after feeding. As the shrimp biomass exceeded 7.5 kg/m, we supplemented additional oxygen at a rate of 0.1~0.3 L/min during conditions in which power outage became cause for concern. In our zero water exchange study, management of super-intensive shrimp culture could not have been done without continual monitoring using YSI 5200 units, which provided real-time DO measurements and subsequently allowed us to use considerably less oxygen than compared to previous trials. During the final week of grow-out, we supplemented oxygen continually at a rate of 0.3~0.5 L/min allowing us to produce quality food-size shrimp with yields as high as 9.75 kg/m.
1.2 Microbial community
The microorganisms associated with this system were characterized using FACS over a 1-month period throughout the middle of the study in order to determine the effectiveness of this technology as a general bacterial monitoring technique. Data identifying gram-positive vs. gram-negative bacteria indicated that the bacterial community present in all four raceways, foam fractionation or settling tank mediated, was dominated by gram-positive microorganisms (
Figure 1). Differences in bacterial community structure were not identified between methods of particulate control or over the characterization period (two-way ANOVA p>0.05).
.png)
Figure 1 Gram-stained bacterial population change determined with flow cytometry |
General communities residing within the tanks including bacteria were characterized and found to consist of six independent populations based upon two auto-fluorescence parameters (red and green). Two-way ANOVA tables for each of the six community members with respect to treatment and week are provided. Changes in populations R1, R2, R3, R4, and R5 all indicated significant effect over time as well as displaying interactions between month and particulate control treatment with respect to populations R1 and R4 (p<0.05; (Figure 2).
|

Figure 2 Characterization of fluorescent dependent water particulate
|
Population R1 and R2 increased in prevalence within the community with regards to treatments (Figure 2). Populations R3, R4, and R5 displayed decreasing trends preceded by a peak occurring during the first 3 weeks (Figure 2). Population R6 was found to be least concentrated in the community as well as the possessing the most stable density (p>0.05) while population R1 wasmost prevalent (most likely bacteria due to low fluorescence and small forward scatter value). These six populations were further subdivided into three size categories based upon forward scatter paired with red fluorescence (this specific fluorescence parameter appeared to drive population differentiation). Picoplankton (0.2~2 µm) remained the greatest fraction of the population accounting for no less than 40% of the cumulative population at any sampling date and reached levels as high as 70% (Figure 3).
|
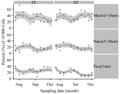
Figure 3 Fluctuation of size dependent biofloc determined with flow cytometry
|
Statistical analysis indicated no differences between FF or ST mediated particulate removal across sampling dates or between each individual population (p>0.05).
In order to try to isolate water quality parameters that influenced the proliferation of microorganisms and particulate in super-intensive zero exchange systems, multiple linear regression analysis was performed with respect to the environmental parameters and flora data. The simplest linear model describing systems with foam fractionation as a solids control method included NO2-N, cBOD5, NO3-N, and turbidity. This model significantly (p <0.05)
according to the adjusted R-squared value. The variability in gram-positive bacteria was negatively influenced by NO2-N (p<0.01) and positively by NO3-N (p<0.05). Turbidity and cBOD5 displayed insignificant negative influence on gram-positive bacteria variability. The variability in macro, nano, and pico particles as well as the variability in autofluorescent regions could not be well described using multiple linear regression models of water quality parameters (p>0.05). The variability of gram-positive.parameters compared to that of foam fractionation. In particular VSS (p<0.05) and cBOD5 (p<0.01) accounted for a majority of the influence. The full model (p<0.01) describing 95% of the variability also included PO4+, TSS, and turbidity. Similarly to foam fractionation, size based particulates as well as autofluorescent regions could not be well described by multiple linear regression models (p<0.05).
Statistical analysis of shrimp harvest data indicate no differences exist in mean final weight between treatments. However, we have shown in support of previous research exist in mean final weight between treatments. However, we observed growth rates varying between 1.35 g/wk and 1.39 g/wk whilst final shrimp weights were 21.9 g and 22.4 g. It is also noteworthy to report that survival rates were high in all four raceways (94.5%~96.8%); FCR was between 1.53 and 1.60. Shrimp yields were 9.34 kg/m to 9.75 kg/m, although the latter weight represented one sample in the FF raceway. The volume of water per 1 kg of shrimp produced used in the raceways varied between 98 L to 126 L.
2 Discussion
We have shown in support of previous researches (
Menasveta, 2002;
Wasielesky Jr. et al., 2006;
Ballester et al., 2010), the efficacy of super-intensive zero exchange systems to produce market-sized
L. vannamei at a very high culture density (450 shrimp/m
3) without the indication of disease. This technique is considered efficient, sustainable, biologically secure, environmentally sound, and therefore an alternative to traditional aquaculture methods, which have notable environmental impacts (
Páez-Osuna, 2001a; 2001b). Unlike other super-intensive zero exchange shrimp rearing studies, we utilized water from a successful 62-day
L. vannamei growth period from larvae to juvenile. We define successful as disease free with low incidence of mortality (<5%). We consider this water to be conditioned with a healthy community of microorganisms due to its success in the postlarvae to juvenile growth period. The bacterial communities associated with marine and brackish
L. vannamei in culture have been shown to change with environment rather than diet (
Luo et al., 2006; 2009) and this result has been reported in fish as well (
Yan et al., 2012). We can infer from these results that the shrimp in our study would obtain a similar beneficial microbial community.
Shrimp culture has been shown to alter the pelagic bacterial community of intensive closed and open culture systems (
Sakami et al., 2008). Therefore, systems utilizing previously conditioned culture water may have a potential advantage over those starting from clean or non-conditioned water in that the initial establishment phase can be foregone resulting in a more stable and complex community of heterotrophic organisms. At the beginning of our sampling period the bacterial community associated with raceways managed by FF and ST solids control were dominated by gram-negative genera. This community transitioned into equal proportions of gram-positive and gram-negative organisms by the end of the grow-out period but transitioned in a polynomial fashion. Gram-negative bacteria generally represent r-selected species whilst gram-positive bacteria represent k-selected or slow-growing species.
Fierer et al (2007) suggest that this delineation is referred to as copiotrophic/eutrophic or oligotrophic, respectively. Utilizing previously conditioned water would provide a possible avenue to maintain a complex culture of these organisms. Nutrient concentrations, particularly with respect to different forms of nitrogen (ammonium, nitrate, urea, and amino acids) tend to significantly impact the proliferation of bacteria in a eutrophic system such as an estuary (
Middelburg and Nieuwenhuize, 2000). A complex community of organisms would therefore increase the ability of the system to handle greater nitrogen loads and result in a more efficient system. We did not explicitly test this idea in our study; however the exemplary growth we report is indicative of excellent culture conditions and these conditions are associated with conditioned water. The re-use of the same water used in the previous nursery trial along with specially formulated feed provided by Zeigler Brothers Inc. may have provided for the immediate establishment or continued maintenance of a healthy nitrifying microbial community in the grow-out study. This allowed for negligible amounts of TAN and NO2-N in the culture water throughout the grow-out study. Typically nitrification will require many weeks to attain a steady state in a closed aquaculture system (
Sohier and Bianchi, 1985), however this system maintained steady state nitrification throughout the growth period. Very few studies have chose to use the aquaculture produced communities of bacteria in pre-conditioned water within enclosed zero exchange RAS. We believe that this avenue of shrimp culture deserves increased attention.
The use of an established community of bacteria will likely prevent the onset of disease through competitive exclusion or limitation of pathogenic strains. We did not observe a single case of disease related death throughout the study. Further, consumers and aquaculturists have become increasingly aware of the hazards of dosing intensively produced aquacultured stocks with antibiotics. Typically, shrimp farmers utilizing this disease prevention technique have incurred crop losses frequently in large production areas such as Thailand (
Holmstrom et al., 2003). The acquisition of antibiotic resistance in potentially pathogenic bacteria such as Vibrio sp. is a typical side effect of antibiotic overuse (
Le et al., 2005;
Nakayama et al., 2006). Further, the use of the antibiotics oxyteracycline and oxolinic acid has been shown to decrease the expression of immune-related genes associated with antibacterial processes in shrimp (
Fagutao et al., 2009). In each of the aforementioned studies, farming practices were carried-out in pond systems rather than RAS.
Probiotic strategies have been employed as an alternative to antibiotics in shrimp aquaculture for the past 20 years; however most of these studies have used a single organism to outcompete pathogenic organisms (
Verschuere et al., 2000;
Kuhn et al., 2010). A change in strategy from individual probiotic cultures to established probiotic communities may be essential to the future of super-intensive growth systems and the continued growth of an environmentally friendly and biologically secure aquaculture industry.
Isolating and culturing a majority of the associated flora of shrimp has proved difficult and therefore research has focused on growth independent identification techniques (
Lau et al., 2002) or those techniques designed to monitor and identify particular microorganisms (
Tendencia and de la Peña, 2001;
Nakayama et al., 2006). We investigated the application of a rapid technique, flow cytometry, as a means to assess changes in bacterial as well as auto-fluorescent pelagic communities of an RAS system. We found that using the gram-stain like procedure developed by
Holm and Jespersen (2003), and initially utilized for milk associated bacteria, the relative densities of gram-negative to gram-positive bacteria could be estimated. We were able to detect weekly fluctuations in this community of bacteria. Specifically, we were able to determine that the two solids control methods employed, foam fractionation and conic cylindrical settling tanks, and did not result in a significant change in the ratio of gram-positive to gram-negative bacteria when using pre-conditioned culture water. The fluctuations we observed within each treatment indicate a level of system homeostasis with respect to gram-negative and gram-positive bacteria. Fluctuations within a system were observed when the ratio of gram-positive:gram-negative bacteria reached 2:8. Gram-positive bacteria cultured from wild habitats (
Rengpipat et al., 2003) as well as produced commercially (
Wang et al., 2005), have received attention regarding their ability to kill or inhibit the growth of gram-negative organisms within marine aquaculture systems. It is therefore critical that a population of these organisms remain within the system to balance potential overgrowth of pathogenic gram-negative organisms. Gram-positive organisms also play an important role in minimizing the accumulation of phosphorous and nitrogen species. Decreased reactive phosphorous as well as total nitrogen and chemical oxygen demand have been reported in systems with gram positive probiotic use (
Wang et al., 2005).
Additionally to bacterial staining, we were able to distinguish three major classes of particles in the biofloc using flow cytometry; pico particles (<2 m), nano particles (2~8 m), and macro particles (7~20 m). Pico particles displayed a range of red fluorescence spanning three log scale regions from 10
0~10
3 relative fluorescence units. Nano and macro particles were found within a more narrow range: 10
1~10
2 and 10
2~10
3, respectively. Although we found that macro particles were in the highest concentration, FACS does not differentiate between single and clustered cells or cells attached to debris. Flow cytometry methods will only detect these conglomerates as a single large “particle”. Macro particles should therefore be considered a diverse group that may require greater resolution. Systems such as closed zero exchange super-intensive culture systems tend to produce suspended aggregates or biofloc. The larger the flocculated material the greater its porosity tends to be as well as it’s potential to interact with the external environment producing a more efficient system. Uptake of nutrients by microorganisms has been found to be more efficient when flocculated than as a single cell (
Logan and Hunt, 1987). It would not be surprising if these particles supported a diverse group of organisms.
The settling tank (ST) particulate control resulted in a slightly decreased but insignificant concentration of nitrate in the respective raceways. This was most likely a product of denitrification by bacteria occurring in the sludge within the STs. In a similar study raising
L. vannamei,
Holl et al (2011) reported that super-intensive systems relying on FF for particulate control resulted in greater accumulation of nitrogen in the system. Further, their study utilized a bead filter method of solids removal, which would produce a denitrifying community of bacteria similar to our STs. Particulate control methods such as settling tank clarifiers have also been demonstrated to promote increased shrimp growth (
Lewis et al., 2009;
Ray et al., 2010). These two groups also suggest that STs and bead filters are more efficient than FF for maintaining adequate water quality with respect to solids and potentially harmful NO
2-N and NH
3. In our study there was a negligible difference between the capacity of the FF and the ST to change the microbial community within the raceway water column when general bacterial community characteristics were used as a proxy. This may have been an effect of using pre-conditioned water.
3 Conclusion
Heterotrophically dominated super-intensive zero exchange greenhouse covered RAS represent an environmentally friendly and biosecure practice for the production of food size L. vannamei. Pre-conditioned water with an established heterotrophically dominated community of microorganisms may serve to provide a more balanced system for the control of harmful nutrients such as NO2-N and NH3-N and help alleviate the risk of disease in these systems. This water may be an alternative to commercial probiotic supplementation and an alternative to individual culture treatments. The solid control methods used in this study behaved similarly unlike in other studies and this may be an effect of pre-conditioned culture water. Flow cytometry served as a cost-efficient high-throughput method for monitoring general community structure of microorganisms within a RAS. Due to the inherent limitations of flow cytometry for community biology we would recommend that future studies be performed in parallel with molecular techniques for a more comprehensive higher resolution investigation of the changes in bacterial communities.
4 Materials and Methods
4.1 Experimental design
A 108-d study was conducted in four 40 m3 (25.4 × 2.7 m with 68.5 m2 bottom area) raceways lined with EPDM (ethylene propylene diene monomer, Firestone Specialty Products, Indianapolis, IN, USA). All raceways were constructed with a longitudinal partition centered over a PVC pipe (5.1 cm long) containing spray nozzles. Six 0.91 m long air diffusers (1.9 cm OD, Aero-TubeTM, Tekni-plex Aeration, Austin, TX, USA) and six banks, each constructed with three 5.1 cm airlift pumps, were positioned at equal distances on both sides of the partition on each of the raceways used in this study. A 2 hp centrifugal pump and a 5.1 cm Venturi injector (model 2081-A, Mazzei Injector Corporation, Bakersfield, CA, USA) served to maintain dissolved oxygen concentrations consisting of atmospheric air or a mixture of 100% bottled oxygen and air. Water for this experiment was obtained from a preceding 62-d nursery study. Four raceways were used in which two were equipped with a commercial foam fractionator (VL65, Aquatic Eco System, Apopka, FL, USA) and the remaining two furnished with a 8.6 m3 cylindroconical settling tank (4.5 m3 working water volume). All four raceways were stocked (450 shrimp/m3) with juveniles (0.99 g ± 0.17 g) of the Pacific White Shrimp, Litopenaeus vannamei, cultured at high density for 62-d in the same tanks. Shrimp were fed seven days a week specially formulated commercial 35% crude protein (CP) feed for intensive systems operated with limited discharge (Hyper-Intensive 35, Zeigler Bros., Gardners, PA, USA). Until Day 18, feed was offered at four equal portions during the day. From Day 19 onward, two-thirds ration partitioned into four equal portions were fed during the day with the remainder fed throughout the night using belt feeders (spring loaded, 12-hour clock 4.5 kg capacity, Zeigler Bros., Gardners, PA, USA). After stocking our tanks in the first week, shrimp feed was mixed with food using in the nursery trial (Fry #4, 30% CP, Rangen Inc., Buhl, ID). Each day rations were calibrated on a FCR of 1:1.4, approximating growth of 1.4 g/wk and assuming a mortality rate of ≤0.5%/wk. Settling tanks (ST) and foam fractionators (FF) were used 23 days after stocking. Both the ST and FF were used intermittently targeting concentrations between 400 to 500 mg/L of culture water total suspended solids (TSS) and settleable solids (SS) between 10mL/L.~14 mL/L. Water flow into the ST varied between 2 and 6 L/min to provide between 4.6 and 13.9 tank turnovers/day. Both the FF and the ST were operated via a side loop, which received the water from the pump installed in each RW. Raceways were maintained with zero water exchange throughout the study. Municipal chlorinated freshwater was added to compensate for water loss due to evaporation and operation of the FF.
4.2 Multiparameter monitoring and statistical analyses
Water temperature, salinity, dissolved oxygen, and pH was simultaneously monitored twice daily using an YSI 650 Series multi-probe (YSI Inc., Yellow Springs, OH, USA). Alkalinity (Method # 2320 B, APHA, 1995), and SS (Imhoff Cone Method # 2540 F, APHA, 1995) were monitored every two to three days. Turbidity (Spectronic 21, Milton Roy Co, Ivyland, PA), TSS (Method # 2540 D, APHA, 1995), volatile suspended solids (VSS) (Method # 2540 E, APHA, 1995), and five-day carbonaceous biochemical oxygen demand (cBOD5) (Method #5210 B, APHA, 1995) were monitored weekly. A Autoanalyzer (dual channel FIAlab-2600, FIAlab Instruments, Inc., Bellevue, WA, USA) was used to monitor weekly total ammonia-nitrogen (TAN) (salicylate method), nitrite-nitrogen (NO2-N), nitrate-nitrogen (NO3-N) (nitrate/nitrite method), and phosphate (water based samples, orthophosphate method). Sodium bicarbonate (160 mg/L as CaCO3) was used to control alkalinity and pH. A YSI 5 200 multi-parameter sonde (YSI Inc., Yellow Springs, OH, USA) was placed in each raceway and data uploaded remotely. Daily and weekly water quality data from each treatment was analyzed using a repeated measures ANOVA. Mean survival, FCR, total yields, weekly growth and final shrimp weights were analyzed using one-way ANOVAs and an value of 0.05. All statistical analyses were conducted using SPSS statistical software (V. 15 for Windows, SPSS Inc., Chicago, IL, USA). Mixing of the ambient air with bottled oxygen did not begin until Day 68 of the study. For a period of 40 days (Days 68 through 108) air was enriched with oxygen only intermittently, specifically for 30~60 minutes following the day-time feeding and only if DO levels dropped below 3 mg/L, at a flow rate of l.0 L/min. During the final week (Day 102 through termination at Day 108), air was constantly mixed with oxygen at a flow rate of 0.3~0.5 L/min.
4.3 Analysis of microbial community
The algal and bacterial communities associated with each raceway were monitored weekly in order to correlate particulate control methods (e.g., FF to ST) to the changes in the populations of algae and bacteria present within the system. Algal monitoring began on July 24 and ended October 2, 2009 while bacterial communities, specifically gram-negative and gram-positive, were monitored only during the final five weeks of the study. Water samples were collected from each raceway in sterile 50 mL conical FalconTM tubes in the morning at ~9:00 AM and transported to Texas A&M University-Corpus Christi to be analyzed within 2 h of collection. A fluorescence activated cell sorter (FACS) flow cytometer VantageSE (Becton, Dickinson and Company, San Jose, CA, USA) in combination with a COHERENT Innova Enterprise II Laser (488 nm) (COHERENT, Inc., Auburn, CA, USA) was used to analyze the water samples. Due to the interspersed use of the FACS, quality control occurred between sampling weeks with BioSure® chicken red blood cells (CRBC) in phosphate buffered saline (PBS) and standard instrument settings to insure that instrument parameters remained within acceptable limits between sampling dates.
Algal communities were distinguished using fluorescence characteristics inherent to the individual cells within (534
±34) nm (FL1) and (630
±22) nm (FL3) fluorescence ranges as well as the forward scatter (FSC) physical parameter, which is indicative of cell size. Prior to analysis, cells were passed through a 100 μm nylon mesh filter to decrease the prevalence of cell aggregates and remove debris. Ten thousand events (one event=one cell passing through the laser path) were acquired for each sample. The greatest community resolution was acquired with the FL1 and FL3 parameters when used on a 4 log-scale plot as well as the FL1 and FSC parameters (
Trask et al., 1982;
Olson et al., 1989).
A procedure analogous to gram-staining was used to determine the percentage of gram-positive vs. gram-negative cells following methods adapted from
Holm and Jespersen (2003). Briefly, a 1.5 mL aliquot of filtered raceway water sample was transferred to a micro-centrifuge tube (~10
6 cells/mL) and centrifugated at 700×g for 10 min. The supernatant was decanted and re-suspended in 1.5 mL of a 3 M KCl solution (3 M KCl, 0.035 M EDTA [pH 7.0], Sigma Aldrich, St. Louis, MO, USA). This solution served to permeabalize the cell wall of gram-negative bacteria. Two hundred and fifty microliters of the suspension was stained with 50 μL of hexididum iodide (HI; 200 μg/mL in 0.1 M NaHCO
3, Invitrogen, Grand Island, NY, USA) and incubated at 50℃ for 15 min. Since HI binds to all bacterial DNA (
Holm and Jespersen, 2003) gram-positive bacteria resist HI staining due to their thicker cell wall. Twenty-five microliters of the secondary stain, Oregon Green 488 nm-conjugated wheat germ agglutinin (WGA; 200 μg/mL in 0.1 M NaHCO
3, Invitrogen, Grand Island, NY, USA), was then added to the suspension and incubated at room temperature for 4 min. WGA binds selectively to gram-positive cells (
Holm and Jespersen, 2003). Ten thousand events were analyzed in the FL1 and FL3 parameters for each sample.
4.4 Statistical analysis
FACS data was analyzed with FlowJo v. 8.8.6 (Tree Star, Inc., Ashland, OR, USA). In order to determine the effects of this system on the biofloc community experiments were carried out in fixed factor three-way ANOVA designs. Solid control method and sampling date were used with microorganism (gram-stain), particle (size) or region (autofluorescence and size) as explanatory variables. The percentage of total microorganisms, particles, or regions in each of these groups was used as the response variables. Data was checked for normality within the scope of ANOVA. In order to determine what water and solids parameters had the greatest impact on the ANOVA results, multiple linear regression models were developed. The percentage of gram-positive and gram-negative bacteria was highly negatively correlated (R2=0.99). Therefore models were created only for gram-positive bacteria. Pairs plots were used in combination with a variance inflation factor (
Heiberger and Holland, 2004) and covariance matrix to remove variables with a high degree of collinearity. Further model simplification was performed using the drop1 function with F-test statistic (
Chambers, 1992). Statistical analyses were performed with R v. 2.10.1 (The R Foundation for Statistical Computing, Vienna, Austria).
Author contributions
JH wrote the manuscript and analyzed the collected data using flow cytometry and statistics. TS designed and supervised the experiment, edited the manuscript, and coordinated the study activities. EC was in charge of the day-to-day operation and management of the experimental system. TM monitored and provided technical assistance. JW monitored water quality in the system. KS aided in flow cytometry, experimental design, and editing of the manuscript and proofs.
Acknowledgement
The authors acknowledge the National Institute of Food & Agriculture (NIFA), USDA, AgriLife Research, National Academy of Sciences, and NOVUS International for their funding. Ziegler Bros. provided feed for the study. Aquatic Eco Systems, YSI, Colorite Plastics, and Firestone Specialty Products provided the foam fractionators, DO monitoring systems, air diffusers, and raceway EPDM liners, respectively used in our study. Post-larvae were supplied by Harlingen Shrimp Farms. Texas A&M University at Corpus Christi provided lab space to perform all flow cytometric analyses for this study. We would also like to acknowledge the staff of the AgriLife Research Mariculture Lab at Flour Bluff, Corpus Christi for their efforts throughout the study.
Reference
Austin B., Stuckery L.F., Robertson P.A.W., Effendi I., and Griffith D.R.W., 1995, A probiotic strain of
Vibrio alginolyticus effective in reducing diseases caused by
Aeromonas salmonicida, Vibrio anguillarum, and
Vibrio ordalii, Journal of Fish Disease, 18: 93-96
http://dx.doi.org/10.1111/j.1365-2761.1995.tb01271.x
Balasubramanian C.P., Pillai S.M., and Ravichandran P., 2004 Zero-water exchange shrimp farming systems (extensive) in the periphery of Chilka lagoon, Orissa, India, Aquaculture International, 12(6): 555-572
Ballester E.L.C., Abreu P.C., Cavalli R.O., Emerenciano M., de Abreu L., and Wasielesky Jr. W., 2010, Effect of practical diets with different protein levels on the performance of
Farfantepenaeus paulensis juveniles nursed in a zero exchange suspended microbial flocs intensive system, Aquaculture Nutrition, 16: 163-172
http://dx.doi.org/10.1111/j.1365-2095.2009.00648.x
Burford M.A., Thompson P.J., McIntosh R.P., Bauman R.H., and Pearson D.C., 2004, The contribution of flocculated material to shrimp (Litopenaeus vannamei) nutrition in a high-intensity, zero-exchange system, Aquaculture, 232: 525-537
Browdy C.L., and Moss S.M., 2005, Shrimp culture in urban, super-intensive closed systems, In: Costa-Pierce B., Desbonnet A., Edwards P., and Baker D., eds., Urban Aquaculture, CABI Publishing, Oxfordshire, UK, pp. 173-186
Chambers J. M., 1992, Linear models, Chapter 4 of Statistical Models, In: Chambers J. M., and Hastie T. J., eds., Wadsworth & Brooks/Cole
El-Haroun E.R., Goda A., and Chowdhury M.A.K., 2006, Effect of dietary probiotic Biogen (R) supplementation as a growth promoter on growth performance and feed utilization of Nile tilapia Oreochromis niloticus (L.), Aquaculture Research, 37: 1473-1480
Fagutao F.F., Yasuike M., Santos M.D., Ruangpan L., Sangrunggruang K., Tassanakajon A., Takahashi Y., Ueno R., Kondo H., Hirono I., and Aoki T., 2009, Differential gene expression in black tiger shrimp, Penaeus monodon, following administration of oxytetracycline and oxolinic acid, Developmental Comparative Immunolology, 33: 1088-1092
Fierer N., Bradfored M.A., and Jackson R.B., 2007, Toward an ecological classification of soil bacteria, Ecology, 88: 1354-1364
Ganguly S., Paul I., and Mukhopadhayay S.K., 2010, Application and effectiveness of immunostimulants, probiotics, and prebiotics in aquaculture: A review, Israel Journal of Aquaculture-Bamidgeh, 62: 130-138
Gómez G.D., and Shen M.A., 2008, Influence of probiotics on the growth and digestive enzyme activity of white Pacific shrimp (Litopenaeus vannamei), Journal of the Ocean University of China (English Edition), 7: 215-218
Heiberger R.M., and Holland B., 2004, Statistical Analysis and Data Display: An Intermediate Course with Examples in S-Plus, R, and SAS, Springer Texts in Statistics, Springer
Holl C.M., Glazer C.T., and Moss S.M., 2011, Nitrogen stable isotopes in recirculating aquaculture for super-intensive shrimp production: Tracing the effects of water filtration on microbial nitrogen cycling, Aquaculture, 311: 146-154
Holm C., and Jespersen L., 2003, A flow-cytometric gram-staining technique for milk-associated bacteria, Applied and Environmental Microbiology, 69(5): 2857-2863
PMid:12732558 PMCid:154518
Holmstrom K., Graslund S., Wahlstrom A., Poungshompoo S., Bengtsson B.E., and Kautsky N., 2003, Antibiotic use in shrimp farming and implications for environmental impacts and human health, International Journal of Food Science Technology, 38: 255-266
Kuhn D.D., Drahos D.D., Marsh L., and Flick G.J., 2010, Evaluation of nitrifying bacteria product to improve nitrification efficacy in recirculating aquaculture systems, Aquaculture Engineering, 43: 78-82
Lau W.W.Y., Jumars P.A., and Armbrust E.V., 2002, Genetic diversity of attached bacteria in the hindgut of the deposit-feeding shrimp Neotrypaea (formerly Callianassa) californiensis (Decapoda: Thalassinidae), Microbial Ecology, 43(4): 455-466
Le T.X., Munekage Y., and Kato S., 2005, Antibiotic resistance in bacteria from shrimp farming in mangrove areas, Science of the Total Environment, 349: 95-105
Lewis B.L., Browdy C.L., Ray A.J., Lawson A., Shuler A., Venero J.A., Vinatea L., and Leffler J.W., 2009, Management of microbial biofloc communities using settling tank clarifiers in superintensive, zero-exchange shrimp production systems, Journal of Shellfish Research, 28: 710-710
Li P., Burr G.S., Gatlin D.M. 3rd, Hume M.E., Patnaik S., Castille G.L., and Lawrence A.L., 2007, Dietary supplementation of short-chain fructooligosaccharides influences gastrointestinal microbiota composition and immunity characteristics of Pacific white shrimp, Litopenaeus vannamei, cultured in a recirculating system, Journal of Nutrition, 137: 2763-2768
PMid:18029496
Logan B.E., and Hunt J.R., 1987, Advantages to microbes of growth in permeable aggregates in marine systems, Limnology and Oceanography, 32(5): 1034-1048
Luo P., Hu C., Xie Z., Zhang L., Ren C, Xu Y., 2006, PCR-DGGE analysis of bacterial community composition in brackish water Litopenaeus vannamei culture system, Journal of Tropical Oceanography, 25(2): 50-53
Menasveta P., 2002, Improved shrimp growout systems for disease prevention and environmental sustainability in Asia, Reviews in Fisheries Science, 10: 391-402
Middelburg J.J., Nieuwenhuize J., 2000, Uptake of dissolved inorganic nitrogen in turbid tidal estuaries, Marine Ecological Progress Series, 192: 79-88
Moss S.M., Reynolds W.J., and Mahler L.E., 1998, Design and economic analysis of a prototype biosecure shrimp growout facility, In: Moss S.M. ed., Proceedings of the US Marine Shrimp Farming Program Biosecurity Workshop, 14 February 1998, Hawaii, United States, The Oceanic Institute, Hawaii, United States, pp.5-14
Moss S.M., 2002, Marine shrimp farming in the Western Hemisphere: Past problems, present solutions, and future visions, In: Lee C.S., and O’Bryen P.J., (eds.), Proceedings of United States. Aquaculture Growout Systems-Challenges and Technological Solutions, Reviews in Fisheries Science, 101(3–4): 601–620
Nakayama T., Ito E., Nomura N., and Matsumura M., 2006, Comparison of Vibrio harveyi strains isolated from shrimp farms and from culture collection in terms of toxicity and antibiotic resistance, FEMS Microbiology Letters, 258: 194-199
Ninawe A.S., and Selvin J., 2009, Probiotics in shrimp aquaculture: Avenues and challenges, Critical Reviews in Microbiology, 35: 43-66
Olson R.J., Zettler E.R., and Anderson O.K., 1989, Discrimination of eukaryotic phytoplankton cell types from light scatter and autofluorescence properties measured by flow cytometry, Cytometry, 10: 636-643
Páez-Osuna F., 2001a, The environmental impact of shrimp aquaculture: a global perspective, Environmental Pollution, 112: 229-231
Páez-Osuna F., 2001b, The environmental impact of shrimp aquaculture: causes, effects and mitigating alternatives, Environmental Mangagement, 28(1): 131-140
Ray A.J., Lewis B.L., Browdy C.L., and Leffler J.W., 2010, Suspended solids removal to improve shrimp (Litopenaeus vannamei) production and an evaluation of a plant-based feed in minimal-exchange superintensive culture systems, Aquaculture, 299(1-4): 89-98
Rengpipat S., Tunyamum A., Fast A.W., Piyatiratitivoraku S., and Menasveta P., 2003, Enhanced growth and resistance to vibrio challenge in pond-reared black tiger shrimp Penaeus monodon fed a Bacillus probiotic, Diseases of Aquatic Organisms, 55: 169-173
Sakami T., Fujioka Y., and Shimoda T., 2008, Comparison of microbial community structures in intensive and extensive shrimp culture ponds and a mangrove area in Thailand, Fisheries Science, 74(4): 889-898
Sandaa R.A., Magnesen T., Torkildsen L., and Bergh O., 2003, Characterization of the bacterial community associated with early stages of great scallop (Pecten maximus), using denaturing gradient gel electrophoresis (DGGE), Systematic Applied Microbiology, 26: 302-311
Solano J.L.O., and Soto J.O., 2006, The functional property of Bacillus for shrimp feeds, Food Microbiology, 23: 519-525
Stokes A., Leffler J.W., Venero J.A., and Browdy C.L., 2009, Developing designing and operating a zero exchange bio-floc based shrimp production system, Journal of Shellfish Research, 28: 732-732
Tendencia E.A., and de la Peña L.D., 2001, Antibiotic resistance of bacteria from shrimp ponds, Aquaculture, 195(3-4): 193-204
Trask B.J., van den Engh G.J., and Elgershuizen J.H.B.W., 1982, Analysis of phytoplankton by flow cytometry, Cytometry, 2(4): 258-264
Vaseeharan B., and Ramasamy P., 2003, Control of pathogenic Vibrio spp. by Bacillus subtilis BT23, a possible probiotic treatment for black tiger shrimp Penaeus monodon, Letters in Applied Microbiology, 36(2): 83-87
Verschuere L., Rombaut G., Sorgeloos P., and Verstraete W., 2000, Probiotic bacteria as biological control agents in aquaculture, Microbiology Molecular Biology Reviews, 64: 655-671
PMid:11104813 PMCid:99008
Vijayan K.K., BrightSingh I.S., Jyapraksh N.S., Alavandi S.V., SomnathPai S., Preetha R., Rajan J.J.S., and Santiago T.C., 2006, A brackishwater isolate of Pseudomonas PS-102, a potential antagonistic bacterium against pathogenic vibrios in penaeid and non-penaeid rearing systems, Aquaculture, 251: 192-200
Wang Y., Xu Z., and Xia M., 2005, The effectiveness of commercial probiotics in northern white shrimp Penaeus vannamei ponds, Fisheries Science, 71(5): 1036-1041
Wasielesky W., Atwood H., Stokes A., and Browdy C.L., 2006, Effect of natural production in a zero exchange suspended microbial floc based super-intensive culture system for white shrimp Litopenaeus vannamei, Aquaculture, 258: 396-403
Yan Q., van der Gast C.J., and Yu Y., 2012, Bacterial community assembly and turnover within the intestines of developing zebrafish, PLoS One, 7(1): e30603
http://dx.doi.org/10.1371/journal.pone.0030603

 Author
Author  Correspondence author
Correspondence author
.png)




